
Box GxP Validation for Life Sciences: Effortless & Efficient
Empowering Box Life Sciences Customers With a Leading-Edge, Data-Driven Validation Platform
Res_Q by Sware transforms and simplifies how life sciences organizations manage Box GxP environments through a revolutionary, data-first approach. Our AI-driven platform doesn't just validate your systems — it gives you complete control over your compliance journey, reducing manual effort, risk, and costs.

Sware Selected as Box’s 2025 Life Sciences Partner of the Year
Unleash Your Box Environment’s Full GxP Potential
Sware’s Compliance Module for Box GxP leverages Res_Q to support and simplify ongoing compliance in individual Box GxP instances.
Res_Q's AI-powered, data-driven platform reduces validation time and costs while providing unprecedented automation and insights. Our technology-first approach means you spend less time on compliance paperwork and more time on core business objectives.
Res_Q integrates seamlessly with your existing system architecture and broader industry digital ecosystem. As your business grows, our platform scales rapidly to accommodate new use cases using a data-first approach that keeps you compliant without slowing you down.
Maintain compliance across Box software releases and your specific configurations with ongoing timely management aligned to Box's current release schedule. Stay up to date with the latest Box features while ensuring audit readiness.
Voices of Success
Innovation that Lowers Risk, Costs, and Delays
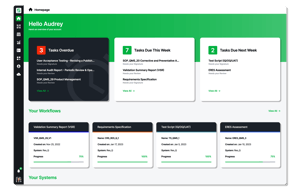
Res_Q creates a fully digital validation ecosystem that automates key workflow elements, maintains superior risk assurance, and drives efficiency at all points in the validation lifecycle, reducing costs and preventing adoption delays.
Initiate workflows and assign workloads based on risk profile. Res_Q puts quality at the forefront without sacrificing speed.
Configurable workflows to both match your operational requirements and integrate industry best practices.
All validation processes are controlled in a single system, serving all areas across the enterprise, including IT, manufacturing, lab systems, and more.
Open API approach unlocks bi-directional data flow that empowers system integrations, migration during onboarding, and more.
