Panelists




Sware

Blue Mountain

SpringWorks Therapeutics



Nathan McBride
Senior Vice President, IT
Xilio Therapeutics
Nathan McBride
Senior Vice President, IT
Xilio Therapeutics
Nathan McBride
Senior Vice President, IT
Xilio Therapeutics
Disclaimer: Statements made by our panelists are their opinions and not affiliated with or reflective of their respective companies.
In today’s rapidly evolving life sciences landscape, traditional approaches to system validation often hinder innovation and efficiency. As technology adoption accelerates, particularly in artificial intelligence (AI) and cloud systems, organizations must adopt agile validation methods to maintain GxP compliance while optimizing operations.
This white paper explores how organizations can transition from project-based validation to a process-driven approach by integrating validation into their Quality Management System (QMS). This integration enables streamlined operations, establishes meaningful success metrics, and fosters sustainable validation practices. Key topics include:
-
The role of vendors as system experts
-
Strategies for writing effective requirements
-
Methods for building cross-functional support
-
Approaches to risk-based decision making
-
Keys to successful QMS integration
The Evolution of Validation in Life Sciences
The life sciences industry is at a critical juncture in system validation. As organizations navigate an unprecedented wave of technological change, categorized by cloud-based systems, frequent updates, and extensive integration, the traditional project-based validation methods are showing their limitations. These methods often create bottlenecks, slowing innovation and operational efficiency.
In response, modern pharmaceutical and biotechnology companies are adopting agile validation practices that prioritize risk management and efficiency while maintaining compliance. By treating validation as an integrated process within the QMS, organizations can promote proactive, sustainable management of validation activities.
Bryan Ennis: “In practice, validation is frequently treated as a distinct project, closely linked to the implementation of a system or significant organizational change. Each time the corresponding SOP is followed, it initiates a comprehensive series of cross-functional activities, many of which may not be required based on the assessed level of risk.”
Validation as a Process
The accompanying infographic illustrates how validation integrates with various quality processes. By viewing validation as a coordinated, ongoing process within the QMS, organizations can promote proactive management of validation activities and deliverables while developing metrics to measure key process indicators (KPIs). This integration is essential for creating a sustainable, efficient validation practice.
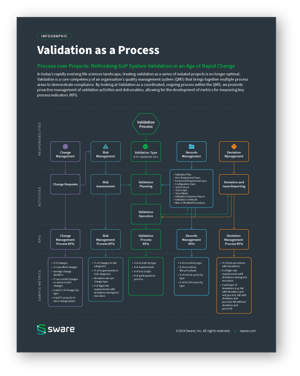
Validation as a Process
The accompanying infographic illustrates how validation integrates with various quality processes. By viewing validation as a coordinated, ongoing process within the QMS, organizations can promote proactive management of validation activities and deliverables while developing metrics to measure key process indicators (KPIs). This integration is essential for creating a sustainable, efficient validation practice.
Bryan Ennis: “With SDLC, we’re once again reentering a paradigm that places emphasis on quality. As we leverage more AI and automated capabilities in engineering and product management processes, we need to focus on data to properly assess product quality and how we govern it. As a Quality leader with an AI background, how do you think about this, in terms of prioritizing quality system design and the kind of controls that need to be in place?”
Aryaz Zomorodi: “I think people are hesitant to move towards AI because of one question: how do you validate something that is ever-changing? You need to have very well-versed individuals that understand how data is coming into this model, how statistical probabilities are being calculated. There’s an ideal reliability score,” but today, there’s no clear-cut way to get there. The regulations are not up to speed yet. As we all know, the paradigm of CSA was developed years ago, and we’re only now getting somewhere with it.”
Process Over Projects: A New Validation Paradigm
The concept of “process over projects” redefines validation as a continuous activity integrated into quality management systems, rather than a series of isolated events. This approach streamlines workflows by aligning validation activities with other quality processes and avoiding unnecessary duplication of effort.
Traditional project-based validation often treats each system change as a standalone project, requiring a complete validation cycle regardless of the change’s scope or risk level. This approach, while seemingly thorough, can lead to significant inefficiencies and lead to “validation debt” – the accumulated burden of excessive documentation and testing that provides little additional value.
In contrast, process-based validation accommodates frequent application changes and allows for a more dynamic response to change. This paradigm recognizes that systems evolve more rapidly today, often with quarterly or monthly updates in cloud environments.
Jennifer Chang: “It’s a lot easier to defend a process during an FDA audit than to defend a series of individual decisions about how you approach different things.”
Integration with Quality Management Systems
The successful implementation of process-based validation depends on seamless integration with the existing QMS. Properly implemented, validation activities align with other quality processes and leverage existing workflows and controls, creating a cohesive approach to quality management.
A modern QMS will accommodate both traditional validation processes and more agile verification approaches. This dual capability allows organizations to apply rigorous controls where needed while handling of routine changes more efficiently. Key steps include:
-
Establishing clear risk assessment frameworks
-
Defining criteria for when full validation is required vs verification.
-
Standardizing change control and documentation templates.
A strong integration enables proactive monitoring, better resource allocation, and a reduction in unnecessary validation overhead.
Aryaz Zomorodi: “Validation is what we do when we first stand the system up, and verification ensures we’re not stuck revalidating every time there’s a change. This agility helps maintain compliance in a fast-paced environment."
Verification vs. Validation: Understanding the Distinction
A critical aspect of modern validation is distinguishing between validation and verification. Validation establishes a system’s foundational compliance, while verification ensures ongoing compliance as changes occur. Well-written requirements are required for both. According to our industry survey, less than 40% of organizations have formal guidance for requirements management. Requirements must be specific enough to ensure compliance while remaining flexible enough to accommodate system evolution.
Industry Survey Poll
Q1: Do you have a policy that governs how requirements are written?

Source: Process Over Projects, Xtalks / Sware Live Webinar Poll; November 14, 2024
Q2: Do you maintain an inventory of the systems your employees use at your CDMO/CROs?

Risk Management and Vendor Partnerships
Vendors play a crucial role in modern validation as subject matter experts (SMEs). Through their interactions with multiple clients, vendors have unique insights into creative and resourceful system uses, best practices for validation, and emerging industry trends.
Critical considerations for vendor management include:
-
Quality Agreements: Establish clear quality agreements that define responsibilities for system verification, disaster recovery, and business continuity
-
System Oversight: Maintain a comprehensive inventory and oversight of all systems managed by external partners
-
Change Management: Define clear processes for managing and reviewing vendor-initiated system changes
-
Communication Protocols: Establish channels for regular updates and emergency notifications
Implementation Strategies and Best Practices
Successful process-based validation begins with a clear understanding of existing systems and processes. Organizations should conduct thorough exercises like SIPOC (Suppliers, Inputs, Process, Outputs, Customers) to ensure that validation efforts align with actual business needs rather than theoretical requirements. Key steps include:
-
Process Understanding: Conduct detailed mapping of current validation processes and their interconnections with other quality systems
-
Stakeholder Engagement: Ensure early involvement from quality, IT, business users, and vendors to build consensus
-
Requirements Management: Develop flexible yet precise requirements that can accommodate future changes while maintaining compliance
-
Training and Support: Build organizational capabilities through comprehensive training and ongoing support programs
Bryan Ennis: “Validation needs to evolve from being a checkpoint to being a continuous partner in quality. By integrating validation into our core QMS processes, we can maintain compliance while enabling the speed and agility modern life sciences companies need. The future belongs to organizations that can turn validation from a barrier into an enabler of innovation.”
Outlook and Industry Trends
The regulatory landscape is evolving, with new guidance emphasizing data governance, vendor and system oversight. Recent FDA and European Medicines Agency (EMA) guidance highlight the importance of adapting quality practices. Organizations must stay informed about these regulatory developments and strategically evolve their QMS to remain competitive.
Emerging trends shaping validation processes include:
-
Regulatory Evolution: Increased focus on data integrity, vendor and system oversight
-
Technology Integration: Growing adoption of AI and machine learning in validated systems
-
Risk-Based Approaches: Increased emphasis on system-specific risk assessment
-
Vendor Ecosystems: Greater complexity in managing multiple vendors
-
Cloud Technologies: Expanding adoption of cloud-based systems and services
Conclusion
The future of validation lies in adopting process-driven approaches that align with modern technological and regulatory landscapes. By embedding validation into quality systems, organizations can achieve greater agility, scalability, and compliance.
Effective implementation requires:
-
Understanding and mapping processes
-
Applying risk-based decision-making
-
Building strong vendor partnerships
-
Investing in training and continuous improvement
By following these principles and adapting them to their specific needs, organizations can develop validation processes that support both innovation and compliance in the modern life sciences environment.

