Life science software validation was a problem that you could control in the past. One or two releases or updates per year used to be manageable, with a predictable amount of IT and Quality resources able to accommodate your needs.
But with the adoption of agile methodology, software companies are generating new features and functions at a higher level of frequency, increasing the pace of SaaS releases, and compounding validation burdens significantly. Evolving compliance regulations (such as Computer Systems Assurance, or CSA), and technology innovations (such as the adoption of AI/ML) have combined with emerging business realities. These realities, such as leaner IT and Quality departments and the ongoing stream of mergers and acquisitions, further increase the software validation challenges life sciences companies face. Companies are struggling to keep up with all these challenges and falling behind on previously unaddressed validation projects, saddling them with an unprecedented amount of validation debt. This situation expressly required a new approach to manage the validation efforts to ensure compliance with all the current laws and regulations.
So, what is validation debt?
Validation Debt (n.) - the accumulation of validation-related imperatives, costs, and bottlenecks an organization faces over its technological lifetime in a heavily regulated industry.
Validation debt adds significant complexity to life sciences compliance, costing companies valuable time, money, and human resources. Outdated validation approaches that use paper-based processes and resource-intensive applications place immense stress on IT and quality leaders, who are now expected to be experts on dozens of clinical and manufacturing systems, regulatory mandates, and personnel management. Ensuring that every person involved has access to specific information about validation procedures becomes critical, as it directly impacts the ability to maintain compliance and streamline operations. The sales pitch to businesses to buy SaaS software usually includes the promise that “it’s easy to be in the cloud”; i.e., that cloud solutions offer easier adoption and maintenance. But in the case of software that needs to be validated, this simply isn’t the case.
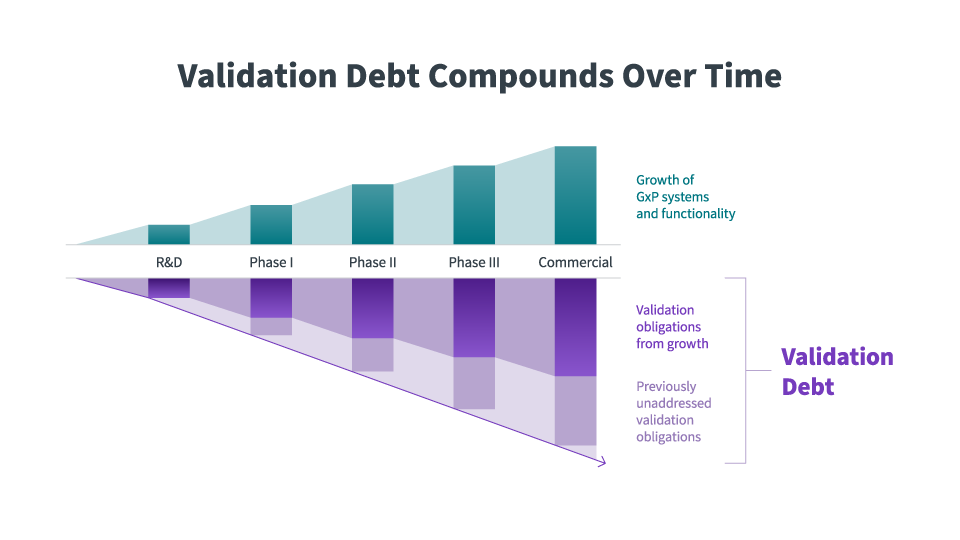
As life sciences companies progress through their clinical and commercial lifecycle, new and complex technologies are adopted, so validation debt continues to compound.
Day-to-day costs aside, inadequate validation methods have serious consequences. Validation quality and documentation gaps expose audit liability, and organizations owe it to their stakeholders to conduct thorough verification of their validation information to prevent these debts from compromising the integrity of their operations. Regulatory red flags can lead to launch delays and massive budget overages, jeopardizing the delivery of new therapies and punishing private and public stakeholders. For smaller pharmaceutical and biotech companies, this isn’t just a bump in the road: it’s an existential threat.
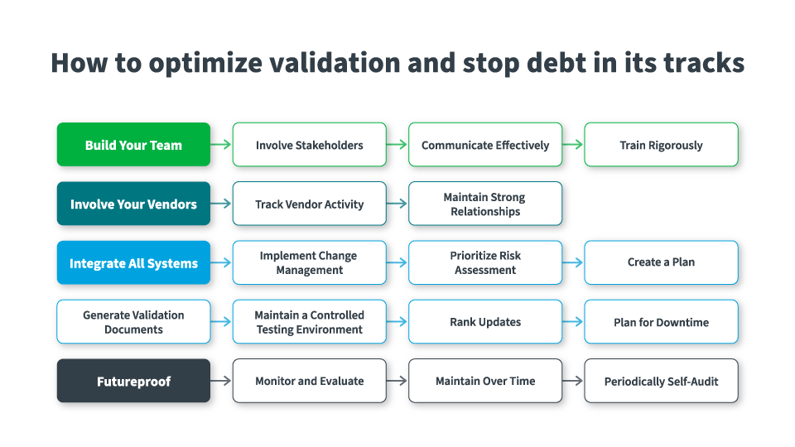
How to optimize validation and stop debt in its tracks
Recognizing the amount of debt tied to validation challenges is the first step toward strategizing on how to stop validation debt. However, mitigating validation debt takes time, strategy, and cross-functional collaboration. Regarding how to reduce validation debt, here are practical steps that organizations can take to draw down their debt, reclaim valuable resources, and mitigate risk:
Build Your Team
- Involve Stakeholders: Engage relevant stakeholders, including end-users, IT staff, and management, in the decision-making process. Encourage input from users and IT staff to classify the risk of new GxP systems and the impact of software updates to existing ones, effectively reducing the debt they're trying to manage and eliminate. Use this feedback to continuously improve the release management process.
- Communicate Effectively: Communicate on the impact of software updates to end-users, IT staff, and other relevant parties. Provide clear instructions, release notes, and information about any changes that may affect them.
- Train Rigorously: Provide rigorous training to end-users and other personnel on the operation of every validated system, ensuring that everyone is equipped to stop validation debt from accumulating further. Effective training ensures that users can interact with requisite systems in a compliant manner.
Involve Your Vendors
- Track Vendor Activity: Stay informed about a software vendor's release schedule, updated policies, and critical security patches. Subscribe to vendor newsletters, mailing lists, or forums to receive timely information.
- Maintain Strong Relationships: Positive relationships with software vendors facilitate open communication, provide access to support resources, and can influence the vendor's responsiveness to specific needs or concerns.
Integrate All Systems to Reach a Debt-Free, Real-Time State of Compliance
One size does not fit all. As you review your validated status, systems will have varying levels of debt and require different levels of validation remediation.
- Effectively Implement Change Management: Take a systematic approach to managing system changes to ensure a state of compliance. Poor change management can cost time and resources down the line, as well as expose your organization to unnecessary risk. Assess General Release notes to understand what system changes are being introduced to best document, assess, plan, execute, and release the system prior to changes being incorporated or deployed.
- Prioritize to Ensure Risk Assessment: Goal and resource prioritization is key. Assess the type of changes you are making to gauge what is important to test, the scope of the test(s), and what can be sufficiently leveraged to reduce validation debt. Determine what resources can be used and where they should be assigned to reach your target goal.
- Create a Plan: Develop a comprehensive validation plan that outlines the efforts to modernize the validation approach and bring the system into real-time compliance. Include criteria for success, test approaches, and how the system will stay in compliance over time.
- Generate Validation Documents: Including a validation master plan, risk assessment reports, and validation protocols. Ensure that these documents are maintained and updated as needed throughout the system’s lifecycle.
- Maintain a Controlled Testing Environment: Before deploying any updates, test them in a controlled setting that mirrors the organization's production environment. This should include functional testing, integration testing, and any other relevant scenarios.
- Rank Updates: Force-rank software updates by the relative level of risk they pose. Critical security updates should be applied promptly, while less vital updates can be scheduled based on the organization's risk tolerance and available resources.
- Plan for Downtime: If the update process requires system downtime, plan for it during periods of low activity to minimize disruption to business operations. Communicate downtime schedules to affected parties in advance.
Futureproof
- Monitor and Evaluate: Monitor systems closely for any issues. Evaluate the success of the update against the criteria defined in the validation plan.
- Maintain Over Time: Implement ongoing maintenance and monitoring procedures to ensure continued compliance and performance of the system. Regularly review and update validation documentation.
- Periodically Self-Audit: Conduct periodic reviews and audits of the system to identify and address any potential issues or improvements. This ensures the system remains in a validated state over time.
Achieving rigorous validation is complex – but it doesn’t need to be
As validation debt compiles, IT and quality leaders face immense pressure to do more with less. Having a sound strategy and actionable goals in place positions your organization for near- and long-term success.
Today, increasingly sophisticated validation solutions offer a seamless, intuitive way to bridge the strategy and actions needed to improve validation processes and eliminate costly validation debt. Res_Q by Sware is purpose-built to address validation challenges across the enterprise. Res_Q automates your validation systems and processes, accelerates validation time by up to 80%, all while ensuring rigorous audit readiness.