Contents:
What Is the Difference Between CSV and CSA?
What is CSV?
CSV stands for Computer System Validation. The goal of CSV is to provide documented evidence that a computer system is fit for its intended use and complies with regulatory standards.
What is CSA?
CSA stands for Computer Software Assurance and represents the FDA’s new guidance towards validation, encouraging risk assessment and critical thinking beyond simply focusing on functionality.
This new approach seeks to stay in line with new technologies, fast-paced software updates, cloud computing and storage and AI.
CSV and CSA can be considered two sides of the same coin, as these are both used in validating software used by life science companies, ensuring proper use according to compliance guidelines and established GxP validation best practices.
It can be said that CSA is the natural evolution of CSV, with the same main goal of validating software and computer systems. CSA, prioritizes a critical thinking-based approach that aims to replace mounds of useless printed screenshots and documentation with risk-based analysis, producing reduced - but far more meaningful - evidence that the validated system meets regulatory requirements for product quality, patient safety, data integrity, and security.
This “thinking-and-analysis first” approach rests in sharp contrast to “old school” CSV, which puts brute force documentation first and thinking last. CSA cuts to the chase and helps companies get down to the essential questions:
- Does the system have a direct or indirect impact on patient safety?
- Does it have a direct or indirect impact on product safety and/or quality?
- Does the system have a direct or indirect impact on data integrity?
When companies plan, assess risk, and put critical thinking first, they are then prepared to develop appropriate validation plans and records. They can:
- Find out what really needs to be assured (through vendor evaluation, risk assessment, and periodic risk reviews)
- Determine the features, operations, functions, and workflows plus their impact on the patient and product to justify the testing level selected.
Although the documentation deliverables of CSA vs. CSV may be the same (validation plans, validation protocols, and test cases), CSA-based deliverables will be shorter, more focused, and more meaningful.
CSA and CSV Differences
Consider how CSA and CSV differ regarding regulations, documentation and use of resources.
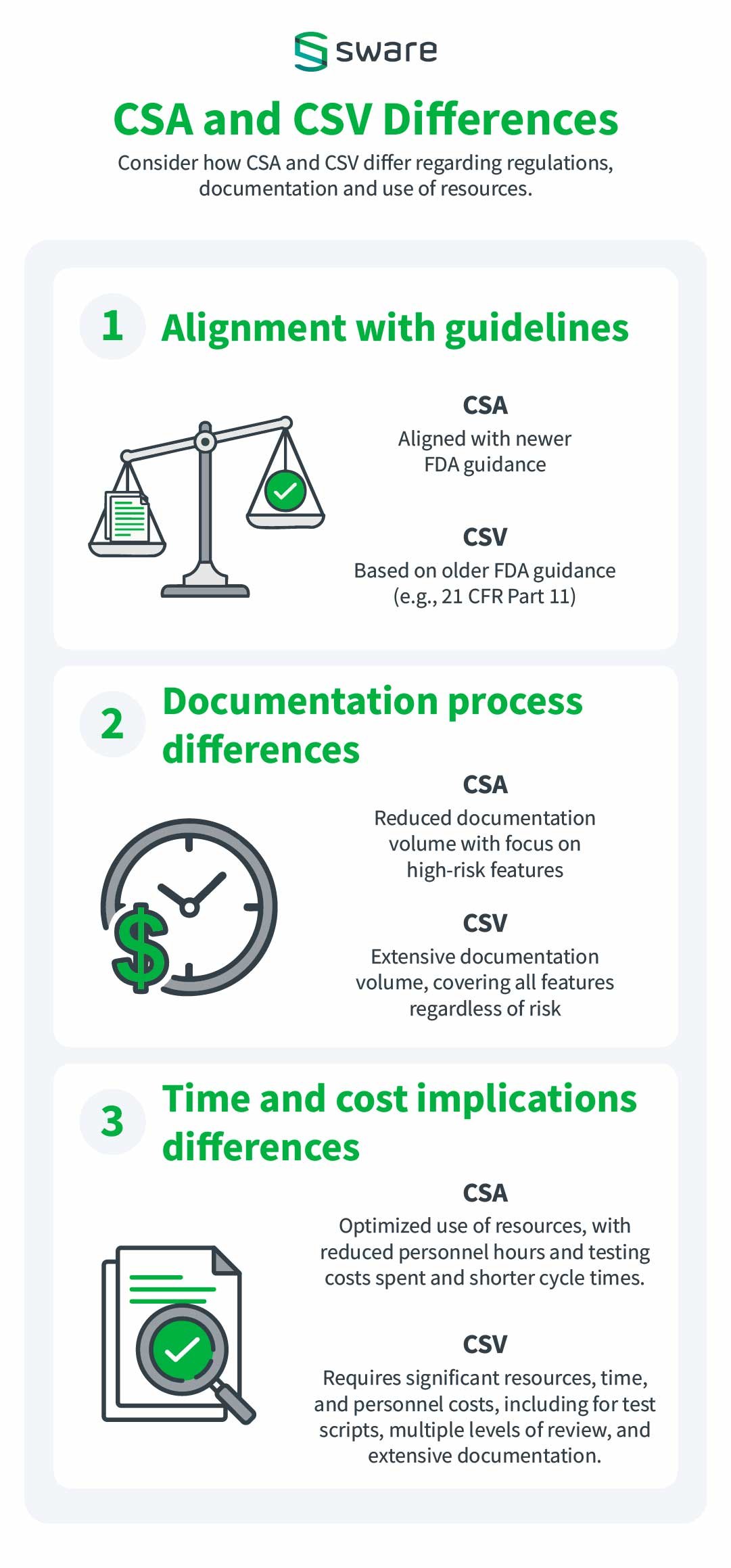
1. Regulatory Perspective Differences
CSA
- Alignment with Guidelines: Aligned with newer FDA draft guidance emphasizing critical thinking and risk assessment.
- Regulatory burden: Reduced, with regulatory activities proportionate to identified risks.
- Focus: Risk-based, focused on critical areas impacting safety, quality, or data integrity
CSV
- Alignment with Guidelines: Based on older FDA guidance (e.g., 21 CFR Part 11) focusing on validation processes.
- Regulatory burden: High, with extensive documentation and testing for all features to meet regulatory expectations.
- Focus: Compliance-driven, often for audit readiness
2. Documentation process differences
CSA
- Volume: Streamlined, focusing only on high-risk features
- Detail Level: Fit-for-purpose, evidence collected only for critical or failed steps
- Redundancy: Reduced, leveraging prior supplier documentation and assurance activities
- Efficiency: Optimized for efficiency with minimal yet meaningful documentation
CSV
- Volume: Extensive, covering all features regardless of risk.
- Detail Level: Detailed, step-by-step protocols with evidence for all steps.
- Redundancy: Increased, often duplicating supplier-provided validation
- Efficiency: Time-consuming and resource-intensive due to unnecessary detail
3. Time and cost implications differences
CSA
- Testing costs: Lower, as testing is tailored to risk levels, using unscripted methods for low-risk features.
- Resources: Optimized use of resources, reducing personnel hours spent on non-critical tasks.
- Cycle times: Shorter due to streamlined processes, unscripted testing, and efficient documentation.
- Overall cost: Lower, as efforts are focused on critical areas, reducing unnecessary steps.
- Workflow methodologies: Consistent with Agile methodologies
CSV
- Testing costs: Elevated due to exhaustive scripted testing of all features.
- Resources: Resource-intensive, requiring significant personnel time for creating, reviewing, and managing documentation.
- Cycle times: Longer due to detailed test scripts, multiple levels of review, and voluminous documentation.
- Overall cost: Higher, driven by excessive documentation, redundant efforts, and comprehensive testing for all features.
- Workflow methodologies: Waterfall-style workflow.
In Summary: What Are the Advantages and Limitations of CSA vs CSV?
Advantages
CSA
- Focuses on critical thinking and risk-based assurance, reducing unnecessary documentation.
- Streamlined processes save time and resources.
- Encourages innovation through iterative approaches (e.g., Agile, unscripted testing).
- Maximizes the use of supplier-provided validation and automation tools.
- Aligns with modern technological advancements like SaaS, AI, and cloud-based systems.
- Reduces cost by minimizing redundant or low-value validation activities.
CSV
- Comprehensive testing for all features ensures extensive compliance documentation.
- Long-established methodology with clear guidance.
- Useful for highly regulated industries with legacy systems requiring exhaustive testing.
Limitations
CSA
- Limited adoption in some organizations due to lack of familiarity or hesitation to shift from traditional CSV methods.
- Transitioning from CSV to CSA requires upfront investment in training and process changes.
CSV
- Resource-intensive: Requires significant time, personnel, and costs for exhaustive testing and documentation.
- Often creates unnecessary documentation for low-risk features.
- Rigid structure with limited flexibility, unsuitable for modern, fast-paced software development approaches.
- Duplicates supplier validation efforts, increasing costs.
- Not optimized for leveraging automation tools.
Who Needs CSA? 10 Questions To Ask Before Transitioning
Is your company adopting new technologies or software at a rapid pace?
CSA's flexibility and adaptability make it well-suited for companies that frequently implement new systems while staying compliant with process validation.Are you looking to reduce the time and cost associated with software validation?
CSA's streamlined approach can lead to significant savings compared to traditional CSV, optimizing the allocation of resources to focus on more important tasks.Do you want to improve your company's risk management practices?
CSA's focus on risk assessment helps companies prioritize their validation efforts and allocate resources effectively. The CSA approach provides much more meaningful insights that can truly help you understand the risks and benefits of implementing certain software.Are you struggling to keep up with evolving regulatory requirements?
CSA's alignment with modern regulatory guidance ensures that companies stay compliant without struggle.Is your current CSV process inefficient or overly burdensome?
CSA offers a more efficient and agile alternative to traditional CSV.Do you want to foster a culture of innovation and critical thinking within your organization?
CSA's emphasis on critical thinking and problem-solving encourages a proactive approach to compliance, focusing on what really matters instead of just meeting regulatory requirements.Are you interested in leveraging automation and new technologies to improve your validation processes?
CSA is compatible with automation tools and innovative technologies, often leveraging external GxP compliance services, which can streamline validation and reduce manual effort.Does your company operate in a highly regulated industry?
CSA can help companies in regulated industries maintain compliance while optimizing their validation processes.Are you looking for a more collaborative and flexible approach to software validation?
CSA's adaptable framework allows for greater collaboration between different teams and stakeholders, leveraging documentation from different sources to avoid duplicating processes.Do you want to ensure that your software systems are reliable, safe, and secure?
CSA's risk-based approach prioritizes critical aspects of system functionality, ensuring that software meets high standards for quality and safety.If any of these questions apply to you, the time has come to implement a CSA-optimized solution that streamlines your validation processes. Res_Q allows biotech and life-sciences organizations to stay in line with newer regulations and their updates while automating processes and reducing the paper-bound documentation that CSV typically entails.
A Real-World Success Story
“Res–Q has saved us significant time and resources, allowing us to focus on core business objectives and reducing validation time by 30-40%. I wholeheartedly recommend Res–Q to fellow industry professionals seeking to enhance their validation practices.” – Senior Director, IT Business Partner, GXP Systems
This case study tells the story of a growing biotech company on a mission to develop groundbreaking treatments for cancer and infectious diseases. By utilizing best-in-class technologies and delivery systems they have developed novel treatments that will revolutionize patient care. Learn more about this success story.
An old validation process that:
-
Lacked centralized control over multiple sites
-
Based on “digital paperless” data housing, and required manual file and resource exchange
-
Required a manual review of assets and documentation to meet timelines and deliver crucial risk assurance
-
Buckling under the growing volume of required validations
Res_Q, the leading-edge, data-first validation management platform
-
A single, SaaS-based application that: bridges validation gaps and offers a single point of control
-
Workflow automation that expedites and prioritizes curation, review, and approval of key documentation
-
API-driven, data-first architecture that interfaces with existing GxP file storage architecture
-
Transparency, speed, and connectivity to increase end-to-end visibility and mitigate risk
-
30-40% reduction in total validation time
-
Reduced costs, improved efficiency, and easy collaboration across multiple sites
-
Increased traceability and audit/compliance-readiness
-
IT and Quality: increased focus on high-value, revenue-generating initiatives
Will AI Change CSV?
AI will fundamentally change CSV and CSA, just as it is transforming many other work aspects. However, it's crucial to remember that AI should be a tool to assist humans in making processes faster and more efficient, not a replacement for them.
To guarantee compliance and success in CSV and CSA, any AI implementation must be accompanied by human QA. This ensures that the generated data aligns with previous results and regulatory requirements.
If you are interested in AI applications in GxP, please watch our webinar.
The Bottom Line
When comparing Computer System Validation vs. Computer Software Assurance, CSA directly addresses the most vital concerns regarding system integrity and safety through a risk-based approach.
In the end, CSA is about what is right for your business. The thinking and planning that goes into the project upfront allow you to allocate resources and test where it is needed most – mindful of quality and compliance, with the goal of keeping your GxP applications in a state of control.
Your team is aligned strategically and can work together to ensure that the software systems being validated are reliable, suited for intended use, and preserve data integrity.
Instead of wasting time on low-priority system validation tasks, you can ensure that there are no critical product quality issues and improve the user experience where it matters most. And that’s where the CSA vs. CSV difference becomes the most apparent.
Changing one letter – and changing your thinking – will help your company escape existing or mounting technical debt, saving valuable budget, resources, and time.
.jpg?width=300&name=SWR-xxx_CSAvCSV-Blog%20(2).jpg)